Abstract
BACKGROUND: Chronic kidney disease (CKD) is common in patients with sickle cell disease (SCD). However, the progression of CKD in SCD and factors associated with such progression remain poorly defined. Liquid chromatography mass spectrometry (LC-MS) based quantitative proteomics has become a highly potent method for biomarker discovery due to growing capabilities for broad proteome coverage and good accuracy and precision in quantification.
OBJECTIVES: The purpose of this study was to identify the potential markers associated with CKD progression in patients with SCD using quantitative proteomics.
METHODS: Urine samples were collected from healthy controls and SCD patients with different CKD stages from University of Illinois at Chicago (UIC). Mass-spectrometry analysis was performed on an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) coupled to a Prominence Nano LC (Shimadzu) using the Xcalibur version 2.7.0 (Thermo Scientific). Proteome Discoverer 1.4 and SIEVE 2.1 programs were used for protein identification and label-free quantification. Heavy isotope labeled peptide EDQTSPAPGLR(13C6, 15N) was used as an internal standard for high resolution/selected ion monitoring (HR/SIM) analysis of HGFL. Urinary HGFL protein together wiht creatinine and albumin were also measured by ELISA.
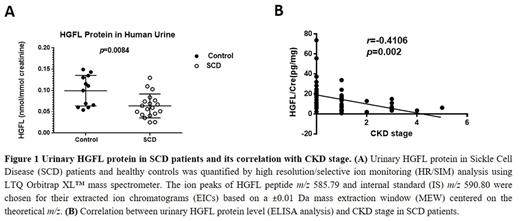
RESULTS: Since glomerular hyperfiltration is an early stage of renal dysfunction. We performed label-free quantitative proteomic analysis for urine samples collected from SCD patients with hyperfiltration (N=3) and normal (N=3). Hepatocyte growth factor-like (HGFL) protein was found to be significantly downregulated (5.52-fold, p=8.05 × 10-5) in samples with glomerular hyperfiltration compared to normal group. Next, we developed a high resolution/selected ion monitoring (HR/SIM) method by measuring the HGFL peptide (m/z 585.79) with isotope labeled-HGFL peptide (m/z 590.80) as internal standard (IS). HR/SIM quantification was performed for 19 urine samples from SCD patients and 12 urine samples from healthy controls. HGFL levels were found to be significantly downregulated (p=0.0084) in the SCD urine samples compared to samples from healthy controls (Figure 1). To further assess the correlation between HGFL level and CDK stage, we expanded the analysis to SCD patients with different CKD stage ranging from 0 to 5 and 19 healthy individuals by ELISA. The result confirmed the finding of HR/SIM quantification, moreover, showed that urinary HGFL level highly correlated with CKD stage (r= ̶ 0.4106, p=0.002, Figure 1) and showed high sensitivity and specificity by Receiver Operating Characteristic (ROC) curve analysis (AUC=0.78).
CONCLUSIONS: HGFL protein has been identified as a negative regulator of phosphatidylinositol 3-kinase (PI3K), and PI3K/Akt pathway was found to be activated in the progress of CKD. Therefore, the decrease of HGFL level in urines from SCD patients may indicate the development of CKD. Combination of LC-MS based quantitative proteomics and ELISA validation is an useful approach for biomarker discovery.
ACKNOWLEDGMENTS: This work was supported by NIH Research Grants 1P50HL118006, 1R01HL125005, 5G12MD007597 and K23HL125984. The content is solely the responsibility of the authors and does not necessarily represent the official view of NHLBI, NIMHD or NIH.
Gordeuk: Emmaus Life Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.